
Prenatal hormones an insignificant contributor to male brain structure
N.E.Whitehead, Ph.D
Journal of Human Sexuality (2014) 6:104-126
The work of (Phoenix, Goy, Gerall, & Young, 1959) and later supporting research, has been taken as evidence that the male brain is prenatally masculinised by testosterone, but only activated to full heterosexual orientation at puberty. This was based on experiments in which testosterone was injected into pregnant guinea-pigs and the female offspring examined, but it has been widely assumed, though perhaps mistakenly, that a prenatal testosterone surge is also the major cause of male sexual orientation in humans. Subsequently, research including studies of the Congenital Adrenal Syndrome (CAS) in girls, has shown multiple influences from a variety of sources on animal/human adult heterosexual orientation and brain structure, making the theory too simplistic. Recent work in the UK (Lombardo et al. 2012), testing the Phoenix et al. theory quantitatively for the first time, is here interpreted to show that the prenatal testosterone effect exists in humans, but at only 16-27% of total influences, so is weak to modest, not major. Two other types of independent calculation in the paper, from twin studies and age of first sexual attraction, support the modest size of the influence. This implies that heterosexuality in humans does not develop solely under the influence of testosterone, but probably requires environmental inputs among which are those from parenting and peers. Homosexuality and transgender orientations have been assumed to be due to prenatal disturbances in testosterone hormone exposure, but the Lombardo et al. result for heterosexuality implies that prenatal influence is at best weak to modest for homosexuality and transgender. The fact that research is increasingly revealing multiple influences, makes parenting important, and sexual therapy a possibility.
Introduction: Experimental work by Phoenix et al.
Hormones are important molecules in cells, and apart from their various regulatory functions in the adult animal, for example sexual function, are involved in the growth and maturation of the fetus. How, and to what extent, these prenatal hormones, particularly testosterone influence actual adult sexual behavior has been unclear, and many theories have emerged with the course of time. The purpose of this paper is to discuss the most popular by Phoenix et al. (1959).
Phoenix et al., (1959) building on work published by others just before the Second World War, exposed pregnant guinea pigs to varying amounts of the male sex hormone, testosterone propionate. The male young seemed little different from controls, but the female young with the highest testosterone doses were “hermaphrodites”, with masculinised genitalia, and little or no lordosis, the submissive posture during mating. Further, adults tended to mount other females as males would, at a 75% rate compared with a rate for female control guinea pigs of 10.5%, that was very significantly different. These effects resulted in some sense in same-sex attraction (SSA) behavior in the guinea-pigs. In hundreds of subsequent papers by others this behavior has been equated with sexual orientation. Although this is dubious because many would say the mental state of the animal is not accessible to observation, this paper allows the equation , but for the sake of review only.
Changed behavior occurred in 6 animals out of one experimental group of 8. This immediately shows that the same treatment had variable effects and did not necessarily create the mounting behavior every time. Similarly the doses sufficient to suppress lordosis in all animals produced hermaphrodites, but if testosterone doses were lower and did not produce hermaphrodites, only 50% of the animals had lordosis suppressed. The control animals developed opposite-sex attraction (OSA), whereas for SSA, the authors commented (p373) “Within each group the effect on lordosis was not related to the quantity of androgen received prenatally” so the SSA production was quite erratic. The point will be relevant to further discussion of SSA later in this paper.
The impression often given by reports of animal work is that complete and reproducible changes of sexual orientation occur with experimental treatment. They rarely do, and there is much overlap in behaviors however produced. Statistical methods must often be used to detect these differences.
The main conclusion of the authors was about OSA, although their work had produced a kind of SSA, and they wrote that there exists (p369) “an organizing or “differentiating” action on the neural tissues mediating mating behavior. During adulthood the hormones are activational.”
The guinea-pigs did not show sexual attraction characteristics when born, and after a period of dormancy sexual orientation only emerged at puberty, i.e. was activated, hence the theory became known as the “organisational-activational hypothesis”. It has been very influential and received at least 950 citations. It fits the universal observation that mammalian young of all species only show any type of sexual attraction well after infancy, around the time of puberty. However there were many answered questions, particularly whether guinea-pig metabolism reflected human metabolism well, and whether the experimental results might have been merely pathological damage to the central nervous system of the animals.
Subsequent Experimental Work
Relatively little further work was done with the guinea pig, because the rat was a more convenient laboratory animal, but it rapidly became clear that the sensitive period for added hormone exposure was a little different in rats, not restricted to prenatal times, but also from just before birth to a few weeks after (McCarthy et al. 2009) and the masculinizing hormone needed was not testosterone but estradiol, a hormone more usually associated with the female reproductive system. This showed that the postnatal influence of hormones could be important for brain development, but this has not been thoroughly investigated for many species. Other work on rats showed there were even strain differences for sensitivity to some sex hormones (McCarthy et al. 2009).
The adult brain differences found in the last several decades, were discussed by (de Vries, 2009). Although anatomical brain sex differences are known for different species, they commented that in most cases we do not understand how, or even whether, these sex differences contribute to sex differences in behavior. In many species, a brain structure called the SDN (Sexually Dimorphic Nucleus) in the Pre-Optic Area (POA), that is part of the hypothalamus, is essential for male behavior in many animals, yet surgical procedures that destroy it in male ferrets, have no effect (McCarthy et al. 2009). Female ferrets have no SDN nucleus at all, but their behavior can still be manipulated with testosterone, casting doubt on whether the brain structure is even relevant in other species. Mice do not have a SDN difference and still show sexual preferences (de Vries, 2009). Any overview would have to highlight the interspecies diversity that is present. This cast further doubt on whether animal models apply to humans.
Similarly showing diversity, (Schulz, Molenda-Figueira, & Sisk, 2009) found evidence in some experimental animals that there was a single extended postnatal sensitive period for steroid-dependent organization of male reproductive behavior that began around birth and ended in late adolescence. This was quite different from guinea pigs, and different from rats. They also found social experience affected both animal brain and sexual behavior. The primate brain seemed rather similar to rat brain in its reactions to sex hormones (Wallen, 2005), but unlike the case for the rat, estradiol was not uniquely important. Some types of masculine behavior seemed completely dependent on maternal socialisation - others independent. So again there were obvious species differences, and some postnatal influence seeming to arise from hormonal effects produced in offspring by maternal grooming. Even so it was unclear how strong this effect was compared with the presumed instinct to reproduce. It might be predicted that although humans might have the same basic hormonal framework, i.e. the importance of steroid sex hormones, the importance of socialisation and learning would be much greater and the influence of hormones reduced. Homo sapiens after all, is the learning animal par excellence.
Although the varied patterns of animal structure/function should have led to caution, it was popularly assumed that the Phoenix et al. findings applied to humans, and fetal testosterone was an overwhelming influence, because a human prenatal testosterone surge was well known (Hines, 2008), and young children were not attracted to the opposite sex until around puberty. This implied a complete cessation of hormonal influence during this time that seems unlikely. However as described by (Byne & Parsons, 1993), even by the mid ‘80s, about fifteen years after the work of Phoenix et al., the academic consensus from all results available was that nature/nurture interaction was the origin of sexual orientation rather than it being exclusively nature or nurture. We must note then, that a belief in the overwhelming importance of either nature or nurture in the scientific literature was not mainstream opinion in the eighties, and still is not.
Human Adrenogenital Syndrome
This section is presented at this point because the work was done a few decades ago and is useful background for current findings. Direct human experimentation is not ethically possible, i.e. testosterone injections into pregnant women. However there is at least one medical condition known for a long time, the Congenital Adrenogenital Syndrome (CAS), shows prenatal sex hormone influence, but its relevance to the Phoenix et al. hypothesis has received little attention.
CAS girls are exposed to about nine times the normal concentration of androgens prenatally through overactive adrenal glands (Wudy, Hartmann, & Homoki, 2000), and are born with masculinised genitalia. They are thus very like the Phoenix et al. guinea pigs given the highest testosterone doses. In the guinea pigs these doses created females with many male behaviors, but in the CAS girls perhaps the most relevant comparison would be the occurrence of any lessened heterosexual orientation, e.g. lesbian /bisexual sexual orientation. A reasonable estimate of the prevalence of this sexual orientation in CAS girls is about 10-20% (and about 10% of CAS girls actually wanted a sex change operation to male) (Whitehead & Whitehead, 2010). This is a much smaller influence than the 75% of female guinea pigs who mounted other females, or the 100% who did not show lordosis, and may be another example of species differences. But these differences suggest other factors besides testosterone may also be important in humans such as upbringing, peer interaction, and cognitive factors.
Current Evaluation of Phoenix et al.
On the fiftieth anniversary of the publication of Phoenix et al, a 2009 issue of the journal Hormones and Behavior was devoted to discussion of the theory. One commentary was (McCarthy, Wright, & Schwarz, 2009) (p1) :
In this time the dogma that has emerged is, simply put, that developmental exposure to gonadal steroids acts on the brain to organize the neural substrate that is then selectively activated in the adult to induce expression of sex specific behavior. This elegant synthesis effectively explained a collection of disparate data and provided a framework against which future work could be read. Evidence in support of the essential truths of the hypothesis have [sic] steadily accumulated over the intervening 50 years, but evidence challenging or refuting the hypothesis has piled up in an equally compelling fashion.”
They also commented how the field needed clarification (p6) “We do not even know what a female brain is other than it is not male”. So the authors deplored undue adherence to the “dogma”.
In that volume, (Diamond, 2009) considered the hypothesis certain for non-humans and almost certain for humans; that seems one of the more extreme positions unless other influences are also admitted. He mentioned that initially the case of transgender people seemed a refutation of the theory, because they were apparently not subject to unusual hormonal conditions in utero, as judged by their typical genitalia, but their brains apparently developed a sexual orientation opposed to their chromosomal status. Therefore Diamond and others advanced a hypothesis that there was a later period in gestation, after the testosterone surge, during which “brain gender” could be fixed or preprogrammed in the brain in a manner different from the earlier time of genitalia development that had needed testosterone. This had been found in primates (Wallen & Hassett, 2009), but there remained no direct experimental support in humans. Results reviewed later in the present paper show in fact there is some contrary evidence for humans. The theory was also incomplete because it did not allow for post-natal hormonal influence, already known for some animal models.
According to a subsequent publication (Semaan & Kauffman, 2010), (p3) for the animal models, the “majority of known sex differences are induced by the sex steroid milieu during early postnatal development “ so this means that most sex differences do not originate pre-natally. It remained unclear to what extent this applied to humans.
Research on New-born Children
According to the original theory, although young children have brains prenatally organised as male or female, they do not express sexual behaviors or attractions until puberty (in 1959 there was little knowledge of postnatal effects of hormones on the brain). However researchers still sought for gender dimorphic behavior in children too young to be affected by parental input. Although there was a slight difference in size between male and female newborn brains, there were very few other differences in actual brain anatomy, and sexual differentiation in brain anatomy seemed to start in a very subtle way only about age 4. It seemed any differences must be at the level of the cell biochemistry, rather than the anatomy though it was also possible that an age of 4 would have allowed extensive socialisation effects.
However some behavioral differences are apparently observed. In the first four days after birth, girls imitate parents faster and more often, and pay more attention to the cries of other babies (Hoffman, 1977). There is a definite difference in sleep/wakefulness maturation that lags in boys (Cornwell, 1993). Newborn girls have a greater sensitivity to electric shock, react more to a puff of cold air on the skin and make more fine gestures, according to (Nagy, Kompagne, Orvos, & Pal, 2007). In all these comparisons the differences are statistical rather than sharply divided by gender, and there is considerable overlap. The two sexes are more similar than contrasting, but the above features provide some very limited evidence for differences in gender behavior that originate in the brain prenatally. Because of possible environmental influence, comparisons made well after birth may be doubtfully valid, and possibly less valid, the later the test.
Human Fetal Testosterone Measurements
The ideal human experiment for comparison with the guinea pigs, would be something like measurement of human fetal testosterone and monitoring the children after birth to see if brain structure at puberty reflected the fetal levels. Results of a program of investigation like this have recently been published, and are now discussed.
Human fetal testosterone can now be measured during pregnancy. Some researchers take advantage of the procedure called amniocentesis, in which samples of amniotic fluid are withdrawn from pregnant women, usually for genetic testing, and researchers, after getting consent, have analysed these for testosterone. The levels reflect those in the fetus and show whether there is a pre-natal surge and its size. Subsequently researchers applied a battery of tests to the children after they were born, to as late as 11 years, investigating whether there was a correlation between testosterone exposure and gendered behavior. There was some Dutch research, but much of the research was done in the Autism Research Unit at the University of Cambridge and the chief researcher was the well-known Simon Baron-Cohen.
Outcome after Birth
In a series of papers over a decade, he and others have shown correlations (though often small) of fetal testosterone with many male-related traits at various ages. These were: correlation inversely with degree of eye contact (Lutchmaya, Baron-Cohen, & Raggatt, 2002a), inversely with greater vocabulary (Lutchmaya, Baron-Cohen, & Raggatt, 2002b), positively with the 2Digit/4Digit finger length ratio (Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer, & Manning, 2004), inversely with empathy (Chapman et al., 2006), positively with autism (Auyeung et al., 2009a; Auyeung, Taylor, Hackett, & Baron-Cohen, 2010), positively with male-type child play (Auyeung et al., 2009b), (but see (Knickmeyer et al., 2005), (van de Beek, van Goozen, Buitelaar, & Cohen-Kettenis, 2009)), positively with hand strength (Lust et al., 2011) positively with some visuospatial ability but not mental rotation (Auyeung et al., 2012), and positively with brain lateralisation, and male-type brain grey-matter features using MRI scans (Mercure et al., 2009), (Chura et al., 2010), (Lombardo et al., 2012), the latter paper being particularly important. Some of these tests were done on 8-11 year old children, a long time after the fetal testosterone measurements. Any results directly measuring sexual attraction have not thus far been published.
A Correlation is Found, but Further Research is Indicated
So one can now test directly whether fetal testosterone correlates with sexually dimorphic anatomical structures, particularly finger length ratios and brain structure (Lombardo et al. 2012). And what is the result? Most importantly, there is a statistically significant correlation between prenatal testosterone exposure and late childhood sexually-dimorphic brain structure , that supports the organizational/activational hypothesis. However the results are rather puzzling compared with previous literature. The authors found three sexually dimorphic brain regions in which the grey matter was proportional to fetal testosterone, but the regions are not those traditionally found in sex-difference research, that generally study the amygdala and the hypothalamus. The authors found the size of the usual sexually dimorphic regions in the amygdala and hypothalamus were not related to fetal testosterone, (perhaps because of postnatal hormonal influence) but on the other hand one region in the amygdala whose size was related to fetal testosterone was not sexually dimorphic. This is not what those in the field would expect, and replication would be reassuring.
Those who have followed similar studies over the last few decades will be hesitant to accept these brain structure results until replicated, because much brain structure work has failed that test. For the purposes of this paper, however the results are tentatively accepted.
Although the authors emphasise that their work shows a link between fetal testosterone and brain regions, they do not think testosterone is the only influence. They invoke later (i.e. postnatal ) androgen surges and epigenetic effects (influences from the environment), also a possible influence from sex hormones made by the placenta. This again shows the current thinking that multiple influences are involved.
Figure 1. The (negative) association between one brain region (grey matter volume of posterior lateral orbito-frontal cortex), and fetal testosterone. Taken from Lombardo et al. (2012). The pale green circle encloses the blue colored area that is the relevant brain region.
Strength of the Correlation
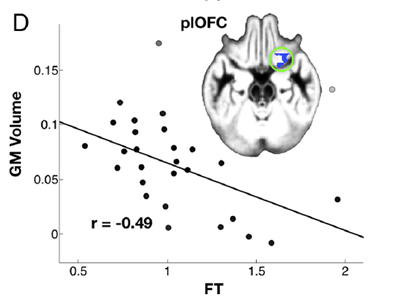
The data scatter, hence the strength of the correlation, is identical for the three brain regions within error. Correlation coefficients range from 0.45 to 0.49 (compared with a maximum 1.0 for perfect correlation), a statistically real effect. However looking at Figure 1, it is clear that there is quite a degree of scatter in the relationship, and probably a lognormal distribution; the strength of the correlation may be overstated. The brain structure grey matter volume is not rigidly dependent on the fetal testosterone. (A similar strength of correlation is found for the finger-length ratios).
To find the degree of influence, or fraction of variance explained, or scatter explained by the regression, one squares that correlation coefficient. That shows only 16-27% of the total variance (roughly total influences) on finger length and brain structure is explained by the fetal testosterone; 73-84% is left unexplained. This makes the fetal testosterone influence weak to modest at best. The authors do not go as far as mentioning that, or other possible implications.
The above calculation used the maximum correlations from the published papers of the Dutch and UK researchers. The other correlations between fetal testosterone and later male traits, like autism, were much weaker or non-existent, therefore many proposed links are not supported by experiment. Another major caveat is that the brain scans were well after the known human six month post-birth testosterone surge that was not assessed by Simon Baron-Cohen and his team. The fetal testosterone association could ultimately prove to be weaker in its effects than the post-birth testosterone association.
An Adequate Test of the Organisational-Activational Hypothesis?
The paper by Lombardo et al. is particularly important because they explicitly put their research forward as a test of the Phoenix et al. hypothesis for the brain – the idea that the “male brain” is fully organised prenatally but activated at puberty. At this point some readers may be puzzled that the testing on the children was done at ages 8-11 that is before puberty. In the text the authors agreed that puberty would be an important area of focus for future work, probably for a more precise test. Two comments should be made. The first is, it was possible that even in late childhood there would be some correlation, and indeed that is what the authors found. This result almost demanded to be published. The second is more complicated – age 8-11 may have been an appropriate age range, because a quite significant literature now maintains that average age of first attraction (hence sexual orientation) to either the opposite sex or same sex is not at puberty, but before puberty at 10 years, however with a very wide age range of several years (Herdt, McClintock, Henderson, Lehavot, & Simoni, 2000). This is “first attraction” that might be merely hero-worship, or a child’s crush on a teacher, but on the other hand could be a real pre-echo of genuine attraction. Lombardo et al. do not explicitly give this as a rationale for testing at ages 8-11. However the age of 10 is conveniently covered by the age range they used. Is it likely that better correlations might be achieved at puberty? We will have to wait and see, but in view of the existence of other influences, that usually tend to reduce correlations rather than increase them, this is not likely. It is also possible that a testosterone/brain structure correlation, as found by Lombardo et al. does not lead inevitably to a particular attraction, (as in the case of the ferrets mentioned previously) so this is another reason the final association between fetal testosterone and attraction may be weaker than expected from the above work, or may not even exist.
Implications of the Modest Correlation with Fetal Testosterone
Some more extreme proponents in the research community may have expected to find an overwhelmingly strong testosterone/brain structure correlation, but in contrast it was surprisingly weak. (Of course it may be that other hormones are important and the focus of attention has been misplaced). This has important implications; although most research finds there is a correlation between fetal testosterone and later maleness, it does not rigidly prescribe male brain structure but only modestly influences it.
The important conclusion is: Heterosexual brain dimorphism seems only modestly prenatally prescribed by prenatal testosterone. We now discuss two other independent lines of research that are consistent with that result.
Other Research Evidence for Modest Influence
The fetal/brain research gave a testosterone influence of 16-27%. There is very little other research that tries to estimate the quantitative strength of prenatal factors on heterosexual attraction, but one paper that did, by (Hershberger, 1997), used twin studies. Although there is some slight doubt if testosterone in the womb is exactly the same even for identical twins, these studies are generally accepted. A weak to modest result (18-26%) for all prenatal factors on OSA was found. The sample was unmarried adults and was unusually favourable for testing heterosexual development; calculations of the genetic influence on OSA from usual whole-population twin samples encounter intractable mathematical problems because relatively few respondents of minority sexual orientations are present. In an unmarried sample the percentage is much higher. Hershberger therefore was able to use this twin study to calculate the total prenatal influence on OSA.
This type of study is particularly important because twin studies test all prenatal factors combined, not just genetics or prenatal hormones, but including any effects of a theoretical later time in gestation when the brain might be pre-programmed as proposed by Diamond and others independently of the earlier testosterone surge.
A result of this modest strength, at the lower end of the range, is similarly found when one investigates the wide spread of ages in first OSA attraction (Whitehead, submitted, see also Whitehead and Whitehead 2010, p34). The wider the age-spread in the appearance of any trait, the less likely it is to be genetic, or biologically programmed, and the large spread for OSA makes the calculated degree of prenatal programming similar to the first two approaches, and a minor factor. These three similar results are shown in Figure 2.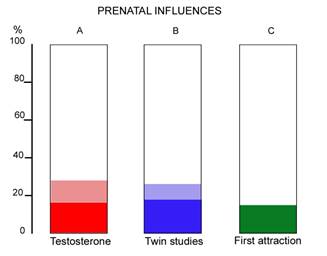
Figure 2. Percentage prenatal influences on OSA as shown by(A) testosterone from amniocentesis, (B) twin studies (C) age spread of first attraction. Shaded areas are 95% confidence limits where known.
The twin studies are the summation of all prenatal influences on adult sexual orientation. The testosterone results are onlythe influence of testosterone on sexually dimorphic brain structure, and therefore more restricted. (For the purposes of this paper it is of course assumed that adult sexual orientation is related to dimorphic brain structure as the Phoenix et al. scheme proposes.) The similarity is important because of the hypothesis of (Diamond, 2009) mentioned earlier, that a prenatal influence independent of testosterone is important for sexually dimorphic brain structure. The similarity of A and B above means that the testosterone entirely accounts for the (quite limited) effects on the brain and there is no room for another influence as posited by Diamond.
We also note that the strength of the masculinising influence for the CAS girls is consistent with the values discussed above (i.e. it was 10-20%). Studies on non-CAS girls from those born following amniocentesis would also be very useful, to understand female OSA, but the Hershberger twin studies indicate any pre-natal influence on females is also weak to modest, like the male results, and it is likely that the influence on female brain structure is similarly very modest.
What does this modest effect mean for homosexual and transgender attraction?
The comparison of A and B in Figure 2 means that there is no room for a late gestation influence on OSA . An unexpected corollary is therefore that the transgender hypothesis of late gestational unusual brain programming as proposed by Diamond (2009). and mentioned earlier, is not very likely – from figure 2, the late gestation period is not significantly involved in establishing OSA in humans and is therefore not likely to be involved in transgender origins either.
There is an independent implication in all this for the development of SSA although only heterosexual orientation was examined in the Lombardo et al. paper. It has been popular to argue that SSA is prenatally programmed in humans and the mechanism for males would probably be a lesser amount of testosterone at critical prenatal times. Such a mechanism with its variable possible amounts of testosterone from slightly deficient to very deficient, would be expected to less tightly correlate with the final brain structure, and therefore have an effect certainly not stronger than the effect on OSA, and more likely weaker. One therefore expects that the association between fetal testosterone and supposed SSA-related brain structure should be less influenced by prenatal hormones than OSA is, so that the effect of prenatal hormones should be weak to modest, at best, not dominant.
Other Factors in Sexual Orientation Development
For the first time we have quantitative evidence about the strength of prenatal hormone influence on sexually dimorphic brain structure. This was expected by many to be overwhelmingly strong for humans, but in contrast it seems testosterone is just one factor among several. Following is a list of some of the other influencing factors found in other research, but in all cases they are not individually predominant. The picture is therefore of many influences of modest strength working together on the brain, not just in fetal life, but after birth through adolescence. Because the multiple factors may interact in many ways and could add or multiply their effects, accurate prediction of final sexual orientation based on these factors is at present too complex.
Research has increasingly revealed many other processes at work in the development of sexual orientation/attraction in humans. Since the time of the Phoenix et al. paper, researchers have discovered that there are some prenatal sexually dimorphic effects in the brain dependent directly on the sex chromosomes (Lenz, Nugent, & McCarthy, 2012), even where there is no influence of sex hormones, and even in disorders of sexual development in which there are no gonads. Estrogen proves essential for feminisation of the brain – females are not simply default males (Lenz et al, 2012). Testosterone masculinises males but there is also an independent process of defeminisation (Lenz et al. 2012). There may be contribution from sex hormones produced in the placenta, and at birth there are high levels of sex hormones in the brain independent of circulating hormones, and made from cholesterol (Konkle & McCarthy, 2011). There is a male testosterone surge in humans just after birth, lasting much longer than the prenatal one, and an estradiol one for females (Winter, Hughes, Reyes, & Faiman, 1976). There is some brain masculinisation after birth (Lenz et al. 2012). Sexually dimorphic human brain changes at puberty seem proportional to the sex hormone levels at that time (Neufang et al., 2009); this implies current hormone levels and not just pre-natal influences are important. Further, since maternal care significantly influences future sexual orientation (at least in rats) (Moore, 1992) there is a general consensus that there are multiple influences at work rather than solely the prenatal testosterone surge.
This leads to a contemporary comment on the “organisational-activational hypothesis” Phoenix paper “…. our current knowledge of sex-based neurobiology has outgrown this simplistic model. Multiple lines of research have contributed to this conclusion” (Reinius, 2011) p15).
For therapists this conclusion should reinforce the idea that therapy in the field of sexual orientation is a possible option. Such therapy will not encounter impassable barriers through brain structures already formed in utero and unalterable.
The conclusion also has possible implications for parents. They cannot merely assume that heterosexuality will automatically develop in their children. As always, guidance and direction are a continuing part of parenting. In In a succeeding paper it is hoped to discuss a further influence on brain structure, death of neurons in a sexually dimorphic way up to adulthood, and unrelated results now available using gene expression measurements for the whole genome, to show the degree of sexual dimorphism in the brain at various ages. These support the interpretation in this paper.
The contribution of prenatal sex hormones to OSA or SSA is not near 100% as many have believed, but is at most about 25%; a minor contribution. In that sense one is not born straight or gay or transgender.
In further summary: the prenatal hormonal contribution to heterosexual brain structure is weak to modest. Similarly, prenatal contribution to homosexual or transgender brain structure is weak to modest.
References
Auyeung, B., Baron-Cohen, S., Ashwin, E., Knickmeyer, R., Taylor, K., & Hackett, G. (2009a). Fetal testosterone and autistic traits. British Journal of Psychology, 100(1), 1-22.
Auyeung, B., Baron-Cohen, S., Ashwin, E., Knickmeyer, R., Taylor, K., Hackett, G., & Hines, M. (2009b). Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychological Science, 20(2), 144-8.
Auyeung, B., Knickmeyer, R., Ashwin, E., Taylor, K., Hackett, G., & Baron-Cohen, S. (2012). Effects of Fetal Testosterone on Visuospatial Ability. Archives of Sexual Behavior, 41(3), 571-581.
Auyeung, B., Taylor, K., Hackett, G., & Baron-Cohen, S. (2010). Foetal testosterone and autistic traits in 18 to 24-month-old children. Molecular Autism, 1(1), 11.
Byne, W., & Parsons, B. (1993). Human sexual orientation. The biologic theories reappraised. Archives of General Psychiatry, 50, 228-239.
Chapman, E., Baron-Cohen, S., Auyeung, B., Knickmeyer, R., Taylor, K., & Hackett, G. (2006). Fetal testosterone and empathy: evidence from the empathy quotient (EQ) and the "reading the mind in the eyes" test. Social Neuroscience, 1(2), 135-48.
Chura, L. R., Lombardo, M. V., Ashwin, E., Auyeung, B., Chakrabarti, B., Bullmore, E. T., & Baron-Cohen, S. (2010). Organizational effects of fetal testosterone on human corpus callosum size and asymmetry. Psychoneuroendocrinology, 35(1), 122-32.
Cornwell, A. C. (1993). Sex differences in the maturation of sleep/wake patterns in high risk for SIDS infants. Neuropediatrics, 24, 8-14.
de Vries, G. J. S. P. (2009). Sex differences in the brain: The relation between structure and function. Hormones and Behavior, 55(5), 589-596.
; Diamond, M. (2009). Clinical implications of the organizational and activational effects of hormones. Hormones and Behavior, 55(5), 621-632.
Herdt, G., McClintock, M., Henderson, A. W., Lehavot, K., & Simoni, J. M. (2000). The Magical Age of 10. Archives of Sexual Behavior, 29(6), 587-606.
Hershberger, S. L. (1997). A twin registry study of male and female sexual orientation. Journal of Sex Research, 34, 212-222.
Hines, M. (2008). Early androgen influences on human neural and behavioural development. Early Human Development, 84, 805-807.
Hoffman, M. L. (1977). Sex differences in empathy and related behaviors. Psychological Bulletin, 84(4), 712-22.
Knickmeyer, R. C., Wheelwright, S., Taylor, K., Raggatt, P., Hackett, G., & Baron-Cohen, S. (2005). Gender-typed play and amniotic testosterone. Developmental Psychology, 41, 517-528.
Konkle, A. T., & McCarthy, M. M. (2011). Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology, 152(1), 223-35.
Lenz, K. M., Nugent, B. M., & McCarthy, M. M. (2012). Sexual differentiation of the rodent brain: dogma and beyond. Frontiers of Neuroscience, 6, 1-12.
Lombardo, M. V., Ashwin, E., Auyeung, B., Chakrabarti, B., Taylor, K., Hackett, G., Bullmore, E. T., & Baron-Cohen, S. (2012). Fetal testosterone influences sexually dimorphic gray matter in the human brain. Journal of Neuroscience, 32(2), 674-80.
Lust, J. M., Geuze, R. H., Van de Beek, C., Cohen-Kettenis, P. T., Bouma, A., & Groothuis, T. G. (2011). Differential effects of prenatal testosterone on lateralization of handedness and language. Neuropsychology, 25(2), 581-589.
Lutchmaya, S., Baron-Cohen, S., & Raggatt, P. (2002a). Foetal testosterone and eye contact in 12-month-old human infants. Infant Behavior and Development , 25, 327-335.
Lutchmaya, S., Baron-Cohen, S., & Raggatt, P. (2002b). Foetal testosterone and vocabulary size in 18 and 24 month infants. Infant Behaviour and Development, 24, 418-424.
Lutchmaya, S., Baron-Cohen, S., Raggatt, P., Knickmeyer, R., & Manning, J. T. (2004). 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Human Development, 77, 23-28.
McCarthy, M. M., Wright, C. L., & Schwarz, J. M. (2009). New tricks by an old dogma: Mechanisms of the Organizational / Activational Hypothesis of steroid-mediated sexual differentiation of brain and behavior. Hormones and Behavior, 55(5), 655-665.
Mercure, E., Ashwin, E., Dick, F., Halit, H., Auyeung, B., Baron-Cohen, S., & Johnson, M. H. (2009). IQ, fetal testosterone and individual variability in children's functional lateralization. Neuropsychologia, 47(12), 2537-43.
Moore, C. L. (1992). The role of maternal stimulation in the development of sexual behavior and its neural basis. Annals of the NY Academy of Sciences, 662, 160-77.
Nagy, E., Kompagne, H., Orvos, H., & Pal, A. (2007). Gender-related differences in neonatal imitation. Infant and Child Development, 16(3), 267-276.
Neufang, S., Specht, K., Hausmann, M., Gunturkun, O., Herpertz-Dahlmann, B., Fink, G. R., & Konrad, K. (2009). Sex differences and the impact of steroid hormones on the developing human brain. Cerebral Cortex, 19(2), 464-73.
Phoenix, C. H., Goy, R. W., Gerall, A. A., & Young, W. C. (1959). Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology, 65, 369-82.
Reinius, B. (2011). Sexually Dimorphic Gene Expression in the Mammalian Brain. Unpublished doctoral dissertation, University of Uppsala, Uppsala, Sweden.
Schulz, K. M., Molenda-Figueira, H. A., & Sisk, C. L. (2009). Back to the future: The organizational–activational hypothesis adapted to puberty and adolescence. Hormones and Behavior, 55(5), 597-604.
Semaan, S. J., & Kauffman, A. S. (2010). Sexual differentiation and development of forebrain reproductive circuits. Current Opinions in Neurobiology, 20(4), 424-31.
van de Beek, C., van Goozen, S. H. M., Buitelaar, J. K., & Cohen-Kettenis, P. T. (2009). Prenatal sex hormones (maternal and amniotic fluid) and gender-related play behavior in 13-month-old infants . Archives of Sexual Behavior, 38(1), 6-15.
Wallen, K. (2005). Hormonal influences on sexually differentiated behavior in nonhuman primates. Frontiers in Neuroendocrinology, 26(1), 7-26.
Wallen, K., & Hassett, J. M. (2009). Sexual differentiation of behaviour in monkeys: role of prenatal hormones. Journal of Neuroendocrinology, 21(4), 421-6.
Whitehead, N. E., & Whitehead, B. K. (2010). My Genes Made Me Do It! (2nd ed.). Lower Hutt, New Zealand: Whitehead Associates.
Winter, J. S., Hughes, I. A., Reyes, F. I., & Faiman, C. (1976). Pituitary-gonadal relations in infancy: 2. Patterns of serum gonadal steroid concentrations in man from birth to two years of age. The Journal of Clinical Endocrinology and Metabolism, 42(4), 679-86.
Wudy, S. A., Hartmann, M., & Homoki, J. (2000). Hormonal diagnosis of 21-hydroxylase deficiency in plasma and urine of neonates using benchtop gas chromatography-mass spectrometry. Journal of Endocrinology, 165(3), 679-83.